Garman Art Conservation Department At SUNY Buffalo State
2020-2023
Attending graduate school during the pandemic had its challenges. However, the Garman Art Conservation Department, my class and the class above and below mine were able to adapt and remain flexible throughout this time. During the first year, lectures were remote with a focus on in-person lab time while the second year allowed for more access to the department.
Projects completed while at Buffalo State were brought to the department by private collectors and local museums, including the Buffalo History Museum and the Onöhsagwë:de’ Cultural Center. Professor Emily Hamilton was my primary advisor throughout my graduate experience. In addition to the objects treatments listed below, I also treated an unvarnished oil painting on paper board and a torn, machine-made, and a handwritten receipt from the 1830’s.
Stained Glass Transom Window
This stained glass from the Buffalo History Museum’s collection was treated during my second year in graduate school and was the subject of my independent study around colorants used in the blue opalescent glass; as seen in the outer arch of this stained glass composition. Fiber optic reflectance spectroscopy (FORS) and x-ray fluorescence (XRF) were investigated to determine their combined effectiveness in characterizing the glass body type and the colorants used in this blue, opalescent, stained glass. The methodology established was designed to be implemented in-situ for the purpose of analyzing stained glass and the identification of possible replacements lights. The eight blue opalescent glass lights that form the arched border and were the subject of this research project are indicated in the normal illumination image below.
Opalescent glass is a semi-opaque glass composed of two or more colors and can have a streaky, cloudy, or mottled appearance. It can either be hand or machine rolled and may be embossed with surface textures, such as the case in this window where there are raised undulating lines mimicking waves upon one side. The mixture of two or more pot-colored glasses in one sheet of glass creates an interesting challenge in the attempt to identify possible colorants due to the differences in elemental intensity. However, the coloring metal oxides proved to be identifiable.
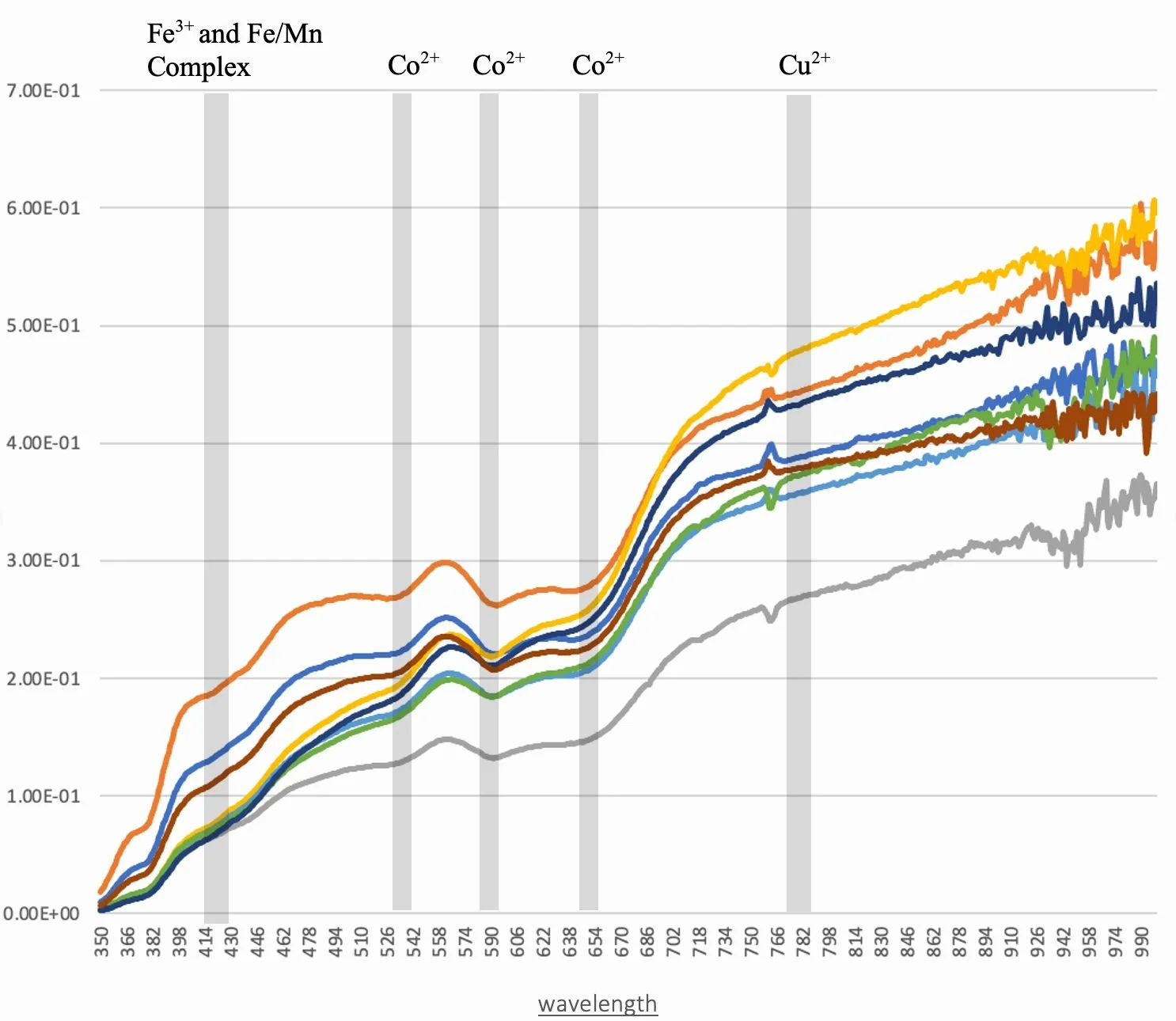
FORS spectral response for all eight of the blue opalescent glass lights.
Beginning with elemental analysis, the Artax Bruker XRF determined the presence of Pb, Ca, Fe, Si, K, Mn, Cu, As and trace amounts of Co, Zn, and Sb in the eight blue opalescent glass lights.
Colorants: Copper oxide will occur in an oxidation reaction and produce a bright green to blue color in glass. A light blue colored glass is observed in the subject of this study leading to the conclusion that this glass was formed in an oxygen rich environment. Cobalt is an incredibly powerful colorant and a low concentration of it will produce a deep blue colored glass; too much and it may be perceived as black and may not transmit much light. When these eight lights are viewed in reflected light they appear a pale blue variegated glass, but when light is transmitted through the glass a distinct yellow hue is visible with streaks of pale blue. A probable culprit of this yellow color in the glass is antimony, as lead antimonate. Arsenic, acts as an oxidizer and was commonly used for clear glass in the 18th century and 19th century. Manganese is present in all four of the glass samples and is also commonly used in the 19th century. Both arsenic and manganese act as decolorizers to reduce the blue-green tint produced by iron inclusions, which are likely present due to the use of sand as the silicate source. Using these decolorizers allowed the glass manufacturer to have a greater control over the color of their product.
Due to the limited range of wavenumbers collected by FORS, the data primarily speaks to the colorant within the glass bodies. The initial impression of these individual lights is that they are incredibly similar, only with slight variations in intensity, but they have similar absorption bands at the same wavenumbers. If it were not already clear by the stained glass fluorescent response, this method would support the claim that none of the eight lights are replacements. Low points, or absorption, visible in the FORS spectrum identifies a detected colorant being present due to the absorption of that wavelength. According to previous research, several known bands can be identified as Co2+, Cu2+ and Fe3+ and Fe/Mn complex bands. These band corroborate with the XRF elemental findings.
Glass Body: Network stabilizers, network formers (a.k.a. fluxes) and network modifiers are the three typical components used in glass manufacturing. Glass with a high amount of lead oxide, greater than 20% of the composition, is referred to as lead glass. The precise percentage of lead was not derived from the XRF data. However, the bright blue color of the eight glass lights in the UVC visible fluorescence image above is consistent with leaded glass, therefore these lights are likely leaded glass. A possible network former is silicon dioxide based on the presence of silicon in the XRF data. A possible modifier for this glass is potassium oxide, which could indicate the use of potash or soda. However, the XRF was unable to detect sodium due to its lighter atomic weight. Therefore, it was not determined if the modifier is potash or soda based on XRF findings alone.
Black ash splint Basket
During Treatment
This plain plaited carrying basket belongs to the Hodinöhsö:ni’. Its methods of fabrication and use are distinct to their cultural traditions. The splints of this basket are likely made from black ash (fraxinus nigra); a cold-tolerant tree native to the rich, wet forested swamps of North America.
Once written and photographic documentation, archival housing was created using corrugated cardboard and PVAc to secure the basket during storage and reduce expose to damaging agents. A tray, of the same construction materials, was fabricated to increase the accessibility of the basket that will facilitate safe handling.
Surface cleaning of the entire basket first required dry brushed and a HEPA filtered vacuum and then followed by cosmetic sponges. The stained splints (darker in color than natural black ash) may have been dyed using black willow and butternut bark dye or walnut and butternut bark dye. These dye of these splints was easily picked up by the cosmetic sponges so the surface cleaning of those splint was limited to the dry brush and HEPA filtered vacuum. During the dry cleaning of the basket the pigmentation of the dyed splints was observed as friable and wet cleaning was not considered to be advantageous due to the potential visual disruptions (tidelines) and loss of pigmentation.
If the original location of a fragment was determined, then the fragment was adhered along the break edges using wheat starch paste. Similarly, fractured splints, with no loss of material, were aligned and adhered along break edges with wheat starch paste. While drying, the breaks were secured using rare earth magnets and silicone release mylar was sandwiched between the magnets and the splint to protect the wood. To use the minimal amount of wheat starch paste the adhesive was only used in areas where a mechanical mend would not be sufficient. A mechanical mend is where no adhesive is introduced and the inherent tension within an object can hold a fill material or joining material in place.
Adhered Japanese paper support (~ 1/8” x 3/8”) only in areas of reattached fragments that were vulnerable of detachment or did not have a large area of attachment and were therefore unstable. Only one support was visible on the inside of the basket and this was toned using PRISMACOLOR® pencils.
Fill materials for the basket are all held in place with tension and are not adhered to the basket. This allows for them to be easily removed if needed without the need of solvents. Evidence of the plaiting pattern based on the degradation of the existing splints informed how to weave the fills. The basket was also determined to be very brittle and therefore the fill material to compensate for the losses needed to be flexible and could not exert too much stress on the existing splints of the basket. Ash splints were tested as a test fill material, but due to the brittleness of the basket it was determined to be too strong. Three types of fills were incorporated into the basket to increase structural stability. For the large, dyed splint forming a horizontal band near the rim of the basket, the large vertical splints and for minor loss in the bottom corners of the basket a thick Japanese Kozo fiber paper toned with QoR watercolors was woven into the areas of loss. For the small, horizontal splints of the sides of the basket, thick Japanese Kozo paper toned with QoR watercolors was woven into the areas of loss. To add to the strength of the paper and to make it a similar thickness of the splint, the paper was stacked three layers thick and adhered together with wheat starch paste. Two of the lengths of paper were the length of the missing section of splint and the third length of paper left long so that it could be woven in front or behind the existing splint. This allowed the fill to compensate for the loss visually as well as structurally by adding a small amount of tension.
The third method of fill was chosen for the loss in the coil around the of the rim of the basket. Smooth Tyvek toned with QoR watercolors was chosen for this fill because of its tensile strength. It has the added benefit of not exerting stress on the paper fills or the brittle weave in the body of the basket. The Tyvek was woven around the four wooden rings of the rim and adhered to itself using wheat starch paste to secure it in position.
Inserted a commercially available bamboo skewer behind one of the vertical splints in the side of the basket that had experienced considerable loss. The wall of the basket was pulling inward toward the center of the basket and the skewer added slight pressure behind the splint, which was enough to keep it in line with the top rim. Not all of the detached fragments were attached to the basket during treatment due to their original location not being determined.Performed written and photographic documentation after treatment.
Special thanks
To the Garman Art Conservation Department, especially my advisor, Emily Hamilton for her guidance with these projects. To the generous financial support contributed by The Andrew W. Mellon Foundation, The Allan Kenzie Family Foundation, The Garman Family Foundation and the National Endowment for the Humanities.